Development of high-performance NF/RO thin film composite (TFC) membranes
Currently, most of the commercial desalination plants apply RO and NF with TFC membranes at the heart of the separation processes. The TFC membranes typically consist of at least two compositional layers, (i) a top, thin selective layer (e.g. polyamide, PA) and (ii) a bottom, porous sublayer (e.g. polyethersulfone, PES). The PA selective layer is fabricated by an in-situ interfacial polymerization reaction between two reacting monomers (diamine and polyacyl chloride), each dissolved separately into an immiscible solvent, at the surface of the porous PES support. When the solutions are brought into contact, the monomers start partitioning across the interface and react to form a thin PA layer at the interface. The porous support provides the required mechanical stability for membrane structure to operate under high pressure while the ultrathin top layer plays the principal role in water filtration. The multilayer feature of TFC membranes exploits the highly desirable advantage that each layer in the composite membrane can be independently optimized with the proper choice of materials and preparation methods for the specific application of interest.
Since the invention of TFC membranes, a significant amount of research and development has been undertaken to modify them in terms of water flux, salt rejection and fouling resistance. The majority of our studies in membrane material development are also focused on the optimization of the interfacial polymerization to develop high-performance TFC membranes. The properties of the thin PA layer (thickness, roughness, and density) depend on the type and concentration of monomers and additives, reaction time and temperature, and post-treatment method. The objective is to provide a clear consensus about the influence of these synthesis parameters on the final properties of the TFC membranes which are used as baseline membranes for the development of thin film nanocomposite (TFN) membranes.
-
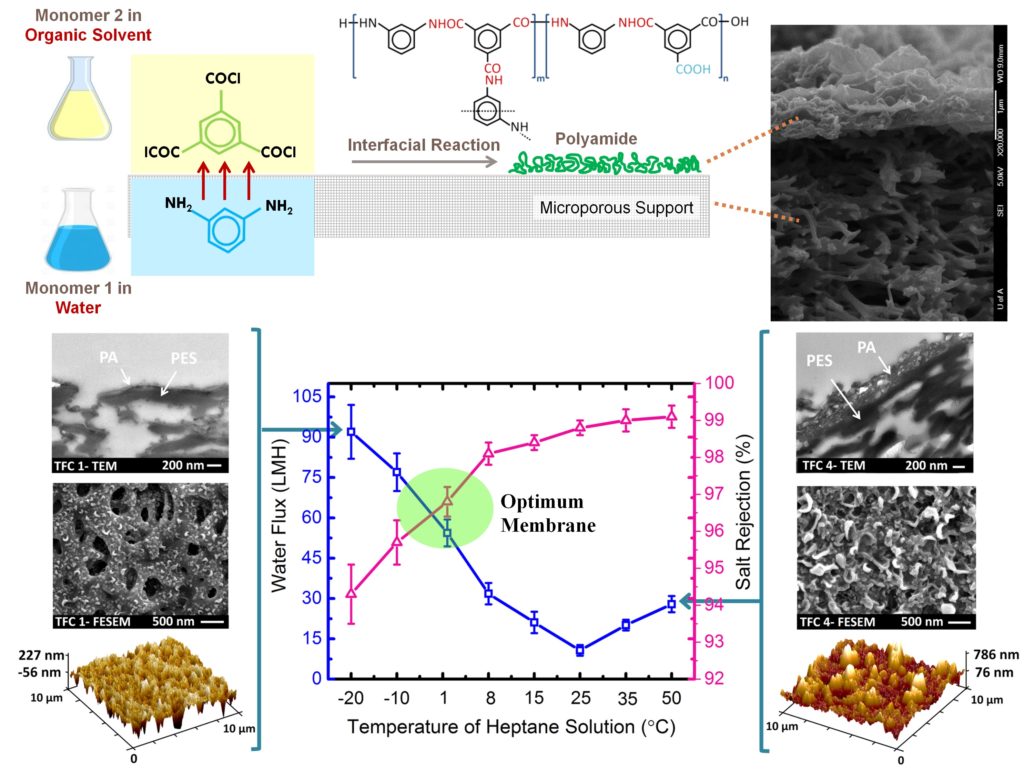
Synthesis of TFC membranes and fully characterization by permeation and microscopy tests
Development of nanocomposite membranes
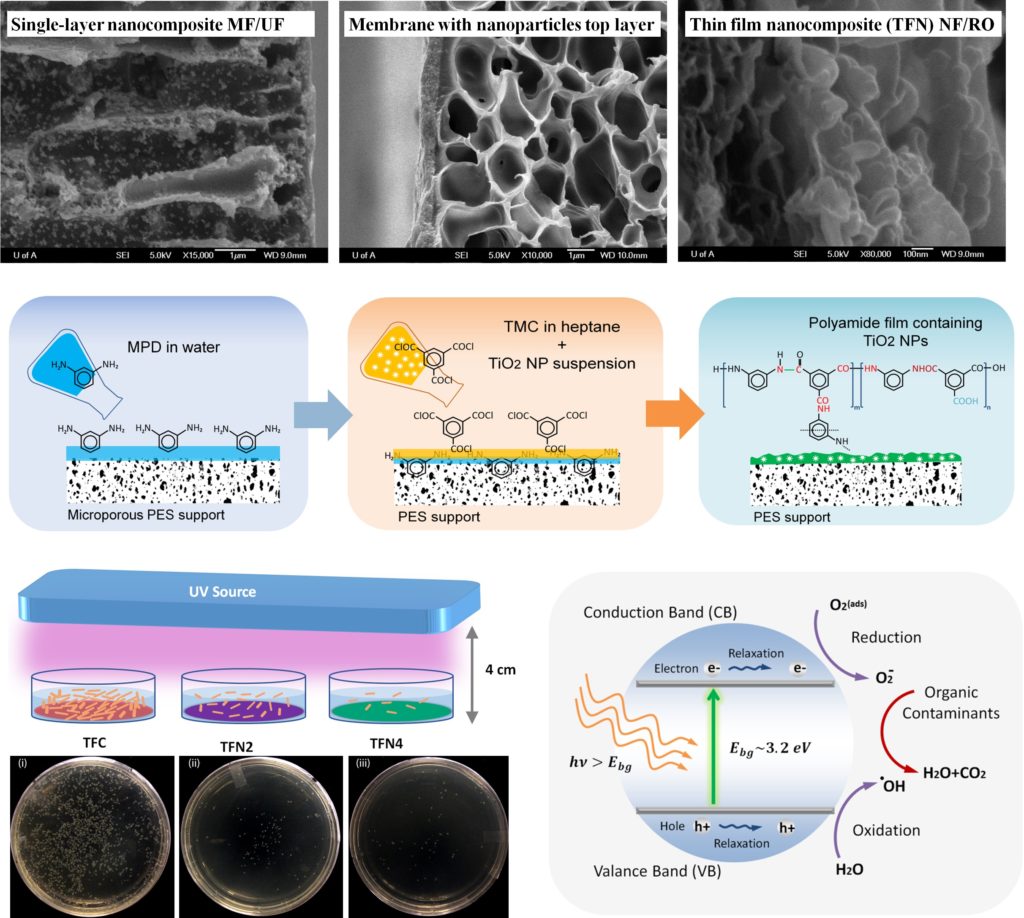
The use of functionalized nanomaterials to change the physicochemical properties of membranes has been widely investigated. The general idea is to induce the thermal, electrical, hydrophilic, anti-bacterial, and molecular sieve properties of these nanomaterials to the base membrane. TiO2 NPs effectively generate highly oxidizing hydroxyl radicals which readily attack and decompose organic contaminants in water. The surface charge of the smart membranes synthesized by the incorporation of ITO and ATO NPs can be tuned by applying an external electrical field to prevent adsorption of foulants based on electrostatic repulsion. Incorporating rod-shaped or tubal TiO2 with a high ratio of length to diameter should improve the thermal stability and anti-compaction properties of membranes more than NPs. Graphene oxide (GO) nanosheets offer an exciting opportunity to integrate the antibacterial, electrical properties and mechanical strength of TiO2, ITO and nanotubes.
The incorporation of nanomaterials to a polymer film, with the aim of fabricating a robust hybrid membrane, may not induce the desired functionality to the polymer and even deteriorate its permeation properties. The major challenges to this are the severe aggregation of the nanomaterials in monomer-containing solutions and the weak compatibility of encapsulated nanomaterials with polymers. For TFN membranes, clusters of NPs cannot be accommodated in a thin PA layer of 100-300 nm, which makes the agglomeration effect even more severe. The non-uniform dispersion of nanomaterials within the host polymer forms non-selective voids at the interface of the polymer and nanomaterials, which significantly reduces the rejection percentage. Also, the desired thermal stability is not achieved, since the predicted reduction in chain mobility by the addition of nanomaterials is mitigated by the formation of these voids. One of the main research themes in our group is to develop strategies for efficient dispersion of nanomaterials in various solvents and membrane materials.
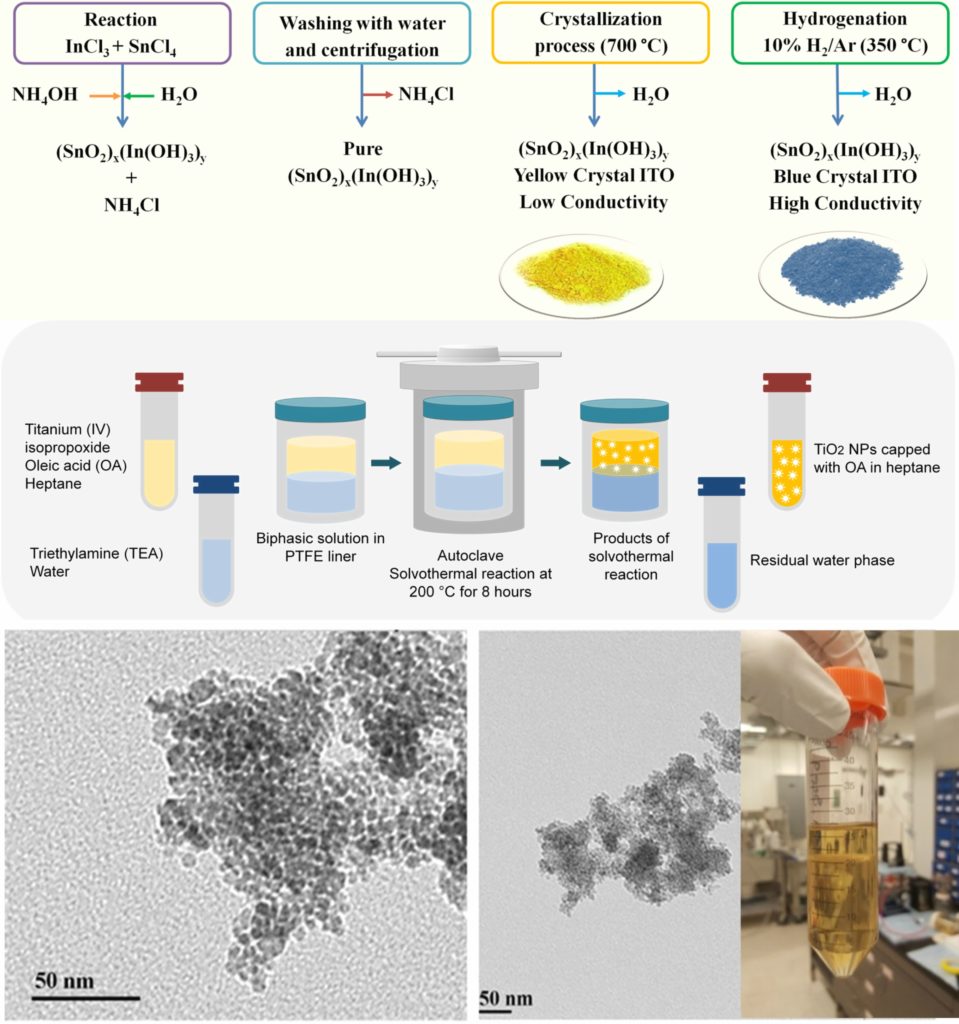
Synthesis of nanomaterials and dispersion study
The incorporation of nanoparticles into polymers to develop advanced nanocomposite membranes was found to significantly enhance properties such as thermal stability, mechanical strength, permeation, and electrical conductivity. Making nano-sized materials and keeping them nano are the major challenges to induce the desired functionality of these materials to the common polymeric membranes.
The proper synthesis of nanoparticles requires a deep understanding of the size and shape of the nanoparticles, as well as the kinetics and thermodynamics of the synthesis reaction. In the AWRL we are able to synthesize TiO2, ITO, and ATO nanoparticles. TiO2 NPs are synthesized using well-known biphasic solvothermal reaction where hydrolysis and nucleation occur at the interface of the organic phase (containing a TiO2 precursor and a dispersing agent) and water phase, resulting in the nucleation of the dispersing agent-capped TiO2 NPs. For the synthesis of ITO and ATO NPs, according to the liquid phase co-precipitation method, their corresponding metal chloride precursors react in the presence of ammonium hydroxide. The product then is crystallized into yellow crystals and hydrogenated to produce blue, conductive crystals.
AWRL is actively collaborating with Dr. Karthik Shankar (Dept. of Electrical Engineering, UofA) and Dr. Pu Chen (Dept. of Chemical Engineering, UWaterloo) to synthesize TiO2 nanowires and nanotubes, and various derivatives of graphene oxide (GO), respectively. The successful application of NPs strongly depends upon the surface modification approaches. For each type of nanomaterial and solvent surface, functionalization strategies must be developed that rely on the balance of intermolecular forces between nanomaterials including attractive forces (e.g. covalent and hydrogen bonding, electrostatic attraction between oppositely charged ligands, and dipole–dipole interactions) and repulsive forces (e.g. steric forces and electrostatic repulsion between ligands of similar charge).